White-tailed Antelope Squirrel
Distribution, Abundance, and Seasonality
The white-tailed antelope squirrel is common to abundant in the deserts of California from Mono Co. south to the Mexican border, and along the northeastern border of California in Lassen and Modoc cos. Optimal habitats are desert scrub, sagebrush, alkali desert scrub, Joshua tree, bitterbrush, and pinyon-juniper. Fairly common in desert riparian, desert succulent shrub, and desert wash habitats. Also occurs in mixed chaparral and annual grassland (Miller and Stebbins 1964, Ingles 1965, Bradley and Mauer 1973, Honeycutt et al. 1981).
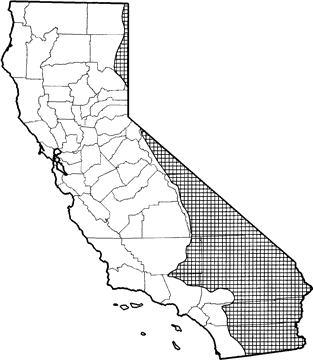
Range Map
Specific Habitat Requirements
Feeding: This omnivore feeds on a variety of seeds, fruits, green vegetation, arthropods, and carrion. In southern Nevada, greens were most important in spring (60% of diet), but less important in autumn (20%); while seeds and fruits were most important in autumn (60%), but less important in spring (20%) (Bradley 1968b). Important plant species include Mormon tea, Joshua tree, opuntia cactus, mesquite, acacia, blackbrush, and grasses. Arthropods formed 30-35% of the diet in autumn (Bradley 1968b). Forages on ground and in shrubs and trees. Carries food in cheek pouches and caches food.
Cover: Simple burrows are dug in friable soil, and are used for escaping predators and extreme temperatures (Grinnell and Dixon 1919, Bartholomew and Hudson 1961, Bradley 1967). Individuals may have several burrows within their home range, and will use abandoned burrows of other animals.
Reproduction: Nesting burrows are under shrubs and in the open, and may have 2 or 3 entrances. The burrow system generally extends less than 0.6 m (2 ft) below the surface. A nest of dried vegetation and hair is constructed (Grinnell and Dixon 1919, Bradley 1967).
Water: Lives long periods without free water, taking moisture from food and minimizing water loss through behavioral and physiological adaptations (Bartholomew and Hudson 1961, Hudson 1962).
Pattern: Prefers open areas in arid and semi-arid habitats; requires friable soil for burrowing. Uses hard-surfaced, rocky, or gravelly soils in open areas with clumps of shrubs.
Species Life History
Activity Patterns: Yearlong diurnal activity. Peak activity is in summer and early fall, and is greatly reduced in inclement weather. Hibernates in the northern parts of its range (Oregon and Idaho), but is active yearlong in Barstow, California. Activity is greatest at temperatures between 15-30¡C (59-86¡F). Depending on time of year, activity may be highest at midday, or in the morning and late afternoon. Surface activity at Barstow begins 1.5 hrs after sunrise and continues until 0.5 to 1.25 hrs before sunset (Karasov 1981). Daily energy expenditure increased from April to October, and metabolic rate dropped at night (Karasov 1981). In winter, reduces energy expenditure by 40% by huddling (Karasov 1983). With a constant photoperiod in laboratory conditions, individuals showed an endogenous rhythm of testicular development, but no circannual rhythms of body mass or water consumption (Kenagy 1981). Changing photoperiod does not affect testicular rhythm, but there is a seasonal difference in tendency to increase body mass (Kenagy and Bartholomew 1979).
Seasonal Movements / Migration: None.
Home Range: Home range estimates in Nevada varied from 1.4-9.4 ha (3-20.6 ac) (Allred and Beck 1963a, Bradley 1967), with a mean of 6.7 ha (14.8 ac) (Allred and Beck 1963a), and a daily fluctuation of 1.8 ha (4 ac) (Bradley 1967). This species can home from distances up to 1.6 km (1 mi) (Bradley 1968a). Densities of 6-35 per km2 (0.4 mi 2) have been reported.
Territory: No data found. Presumably non-territorial. Forms hierarchies in small feeding groups (Fisler 1976, 1977). Huddles in small groups in winter (Karasov 1983) for heterothermy.
Reproduction: Breeding season from February through June with a peak in births in April. The litter size ranges from 5-14, with a mean of 9. Females may have 2 litters per yr, but 1 is considered normal (Grinnell and Dixon 1919, Bradley 1967).
Niche: This small, diurnal omnivore is sympatric with the Mohave ground squirrel, which is competitively superior, but lacks many of the behavioral and physiological adaptations of this antelope squirrel that allow the latter to have yearlong activity (Bartholomew and Hudson 1961). Major predators probably include hawks, owls, coyotes, kit foxes, badgers, bobcats, weasels, and snakes (Grinnell and Dixon 1919).
Sources & References
California Department of Fish and Game, 1999.
California's Wildlife, Sacramento, CA.
Written by: V. Johnson, J. Harris, reviewed by: H. Shellhammer, edited by: R. Duke, S. Granholm
Allred, D. M., and D. E. Beck. 1963a. Ecological distribution of some rodents at the Nevada atomic test site. Ecology. 44:211-214.
Allred, D. M., and D. E. Beck. 1963b. Range of movement and dispersal of some rodents at the Nevada atomic test site. J. Mammal. 44:190-200. Bartholomew, G. A., and J. W. Hudson. 1961. Desert ground squirrels. Sci. Am. 205:107-116. Bradley, W. G. 1967. Home range, activity patterns, and ecology of the antelope ground squirrel in southern Nevada. Southwest. Nat. 12:231-252. Bradley, W. G. 1968a. Homing in the antelope and round-tailed ground squirrels. J. Ariz. Acad. Sci. 5:22-26. Bradley, W. G. 1968b. Food habits of the antelope ground squirrel in southern Nevada. J. Mammal. 49:14-21. Bradley, W. G., and R. A. Mauer. 1973. Rodents of a creosote-bush community in southern Nevada. Southwest. Nat. 17:333-344. Chappell, M. A., and G. A. Bartholomew. 1981. Activity and thermoregulation of the antelope ground squirrel, Ammospermophilus leucurus, in winter and summer. Physiol. Zool. 54:215-223. Fisler, G. F. 1976. Agonistic signals and hierarchy changes of antelope squirrels. J. Mammal. 57:94-102. Fisler, G. F. 1977. Interspecific hierarchy at an artificial food source. Anim. Behav. 25:240-244. Grinnell, J., and J. Dixon. 1919. Natural history of the ground squirrels of California. Calif. State Comm. Horticulture Bull. 7:597-708. Honeycutt, R. L., M. P. Moulton, J. R. Roppe, and L. Fifield. 1981. The influence of topography and vegetation on the distribution of small mammals in southwestern Utah. Southwest. Nat. 26:295-300. Hudson, J. W. 1962. The role of water in the biology of the antelope ground squirrel, Citellus leucurus. Univ. Calif. Publ. Zool. 64:1-61. Ingles, L. G. 1965. Mammals of the Pacific states. Stanford Univ. Press, Stanford, CA. 506pp. Karasov, W. H. 1979. Winter feeding by antelope ground squirrels (Ammospermophilus leucurus): a case of time limitations. Bull. Ecol. Soc. Am. 60:90. Karasov. W. H. 1981. Daily energy expenditure and the cost of activity in a free-living mammal. Oecologia (Berlin) 51:253-259. Karasov, W. H. 1982. Energy assimilation, nitrogen requirement and diet in free-living antelope ground squirrels, Ammospermophilus leucurus. Physiol. Zool. 55:378-392. Karasov, W. H. 1983. Wintertime energy conservation by huddling in antelope ground squirrels (Ammospermophilus leucurus). J. Mammal. 64:341-345. Kenagy, G. J. 1981. Endogenous annual rhythm of reproductive function in the nonhibernating desert ground squirrel, Ammospermophilus leucurus. J. Comp. Physiol. A132:251-258. Kenagy, G. J., and G. A. Bartholomew. 1979. Effects of day length and endogenous control of the annual reproductive cycle of the antelope ground squirrel, Ammospermophilus leucurus. J. Comp. Physiol. A130:131-136. Kram, K. R. 1972. Body temperature regulation and torpor in the antelope ground squirrel, Ammospermophilus leucurus. J. Mammal. 53:609-611. Miller, A. H., and R. C. Stebbins. 1964. The lives of desert animals in Joshua Tree National Monument. Univ. California Press, Berkeley. 452pp.
California Animal Facts | California's Wildlife